OPEN·SYSTEM·700 · MBST therapy device
The proven OPEN·SYSTEM·700 therapy device is designed for the gentle and safe treatment of multiple indications around the musculoskeletal system. For example degenerative diseases, disorders of bone metabolism, acute and chronic injuries.








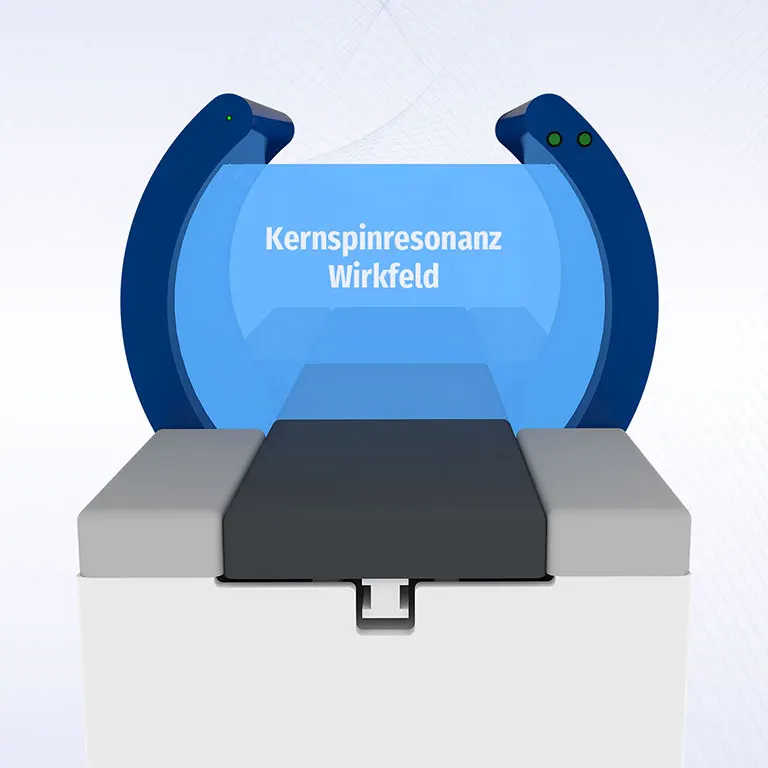












OPEN·SYSTEM·700
The sliding lying surface allows the ideal positioning of physically handicapped patients in the treatment area. Because of the open construction, even claustrophobic and anxious patients can be treated with MBST free of stress.
The patented medical technology of the MBST therapy device complies with current EU norms and can be used for all age groups.
The most important advantages of OPEN·SYSTEM·700 at a glance:
| Max. patient weight: | 120 kg |
|---|---|
| Treatment field: | approx. 150 l |
| Construction: | open therapy device |
Extract of the list of possible indications that have already been treated successfully with MBST magnetic resonance therapy:
Ahlbaeck’s disease, baker’s cyst, bone bruise, bone marrow edema syndrome, bursitis, calcerous tendinitis, chondromalacia patellae, CRPS, femoroacetabular impingement, fractures with and without joint involvement, Hoffa-Kastert syndrome, impingement, jumper’s knee, labrum lesion, lumbago, lumbar radiculopathy, meniscal lesion, microfractures, Osgood-Schlatter-disease, osteoarthritis (coxarthrosis, facet joint arthrosis, gonarthrosis, omarthrosis, osteoarthritis of the sacroiliac joint, polyarthrosis, posttraumatic arthrosis, spondylarthrosis), osteochondritis dissecans, osteochondrosis, osteonecrosis, osteopenia, osteoporosis, partial ruptures of ligaments, muscles, tendons, periostitis, Perthes-Legg-Calvé disease, prosthesis loosening, pseudarthrosis, spinal disc protrusion, spinal stenosis, spondylolisthesis, spondylolysis, spondylosis, stress fracture, tendinitis, tendinopathy, whiplash injury
All technical data contained in this publication is for product information purposes only and not legally binding. In case of further development of products, the older versions will become invalid. The current technical data will be provided by MedTec Medizintechnik GmbH upon request. Please note that technical data states only average values. All values are generated by tests according to industry standards. The results may vary due to different standard interpretations by different service providers or laboratories. All technical data applies to new products only. Deviations due to production may occur.


Information for patients about the therapy device OPEN·SYSTEM·700